
Illustration Of A Amorphous Cells Structure Black And White Layered Artwork.
Amorphous vs. Semi-Crystalline Polymers December 23, 2016 admin No Comments High temperature polymers are divided into two categories: amorphous and semi-crystalline. The difference between the two lies in their molecular structure. In this blog post, we ll discuss how amorphous and semi-crystalline thermoplastics differ from each other.

Crystalline vs Amorphous Difference and Comparison
The difference between the two lies in their molecular structure. Before you decide which to use, you need to understand the characteristics of each, as that will determine your injection moulding process. In this guide, we'll cover: What is an amorphous polymer? What is crystalline polymer? Examples of amorphous polymers

Xrd Crystallinity Hot Sex Picture
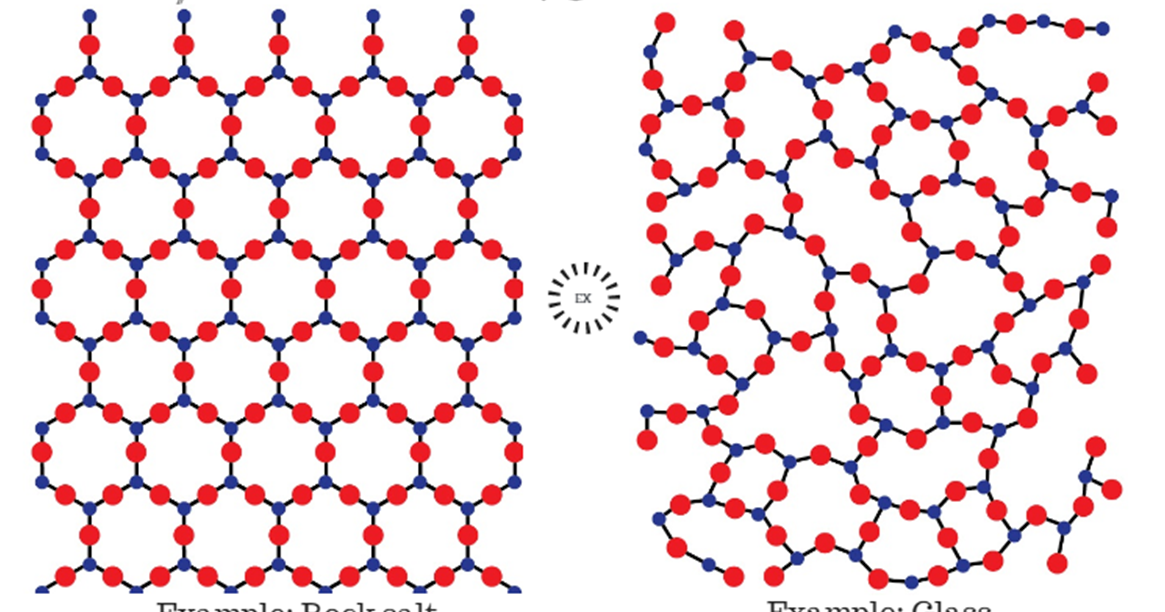
The lattice of crystalline quartz (SiO 2).The atoms form a regular arrangement in a structure that consists of linked tetrahedra. In an amorphous solid, the local environment, including both the distances to neighboring units and the numbers of neighbors, varies throughout the material.

15 Schematic cooling (1) and heating (2) DSC curves, showing a range of... Download Scientific
Most crystalline polymers have amorphous regions, which means crystalline polymers are never completely crystalline.
温度塑料工艺的影响 开云体育登录入口在哪儿下载
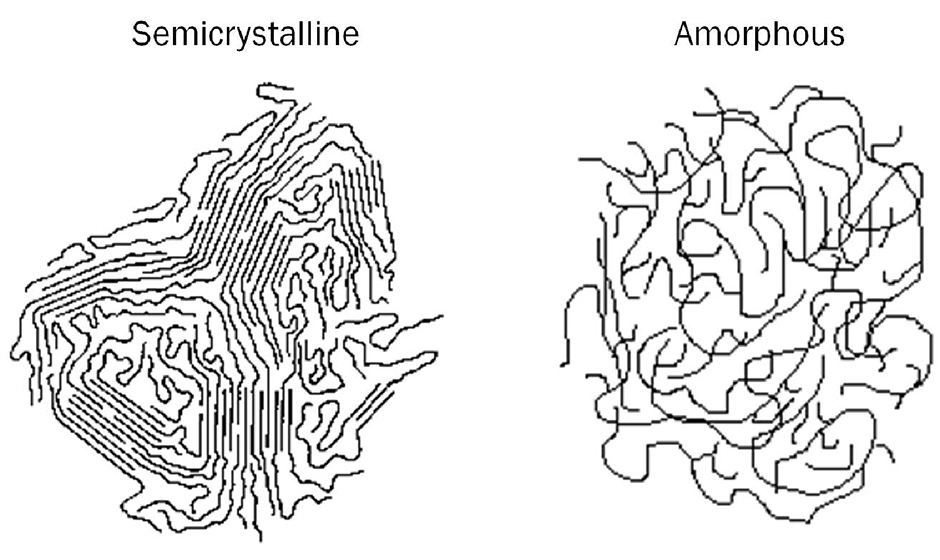
AMORPHOUS POLYMERS - are characterized by having a disorganized pattern of polymers. The polymer chains are disoriented, random in length, and intertwined like a bowl of pasta. Amorphous comes from the Greek word for "shapeless." The random structure has definite advantages in terms of properties.

Difference Between Informative, Experiments, Chemistry
Polymer Crystallinity. To account for the physical differences between the different types of polymers, the nature of the aggregate macromolecular structure, or morphology, of each substance must be considered.Because polymer molecules are so large, they generally pack together in a non-uniform fashion, with ordered or crystalline-like regions, called crystallites, mixed together with.

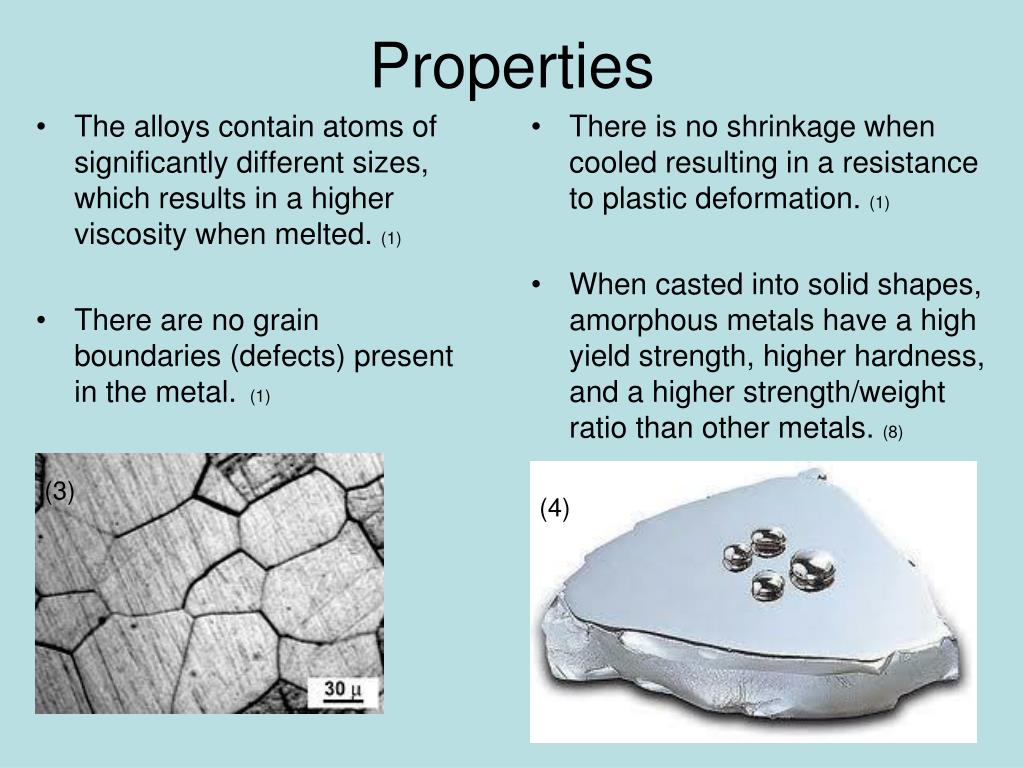
Metal Powder Specialist Heraeus Printed 2 kg Amorphous Alloy Gears in 3D SUM provide popular
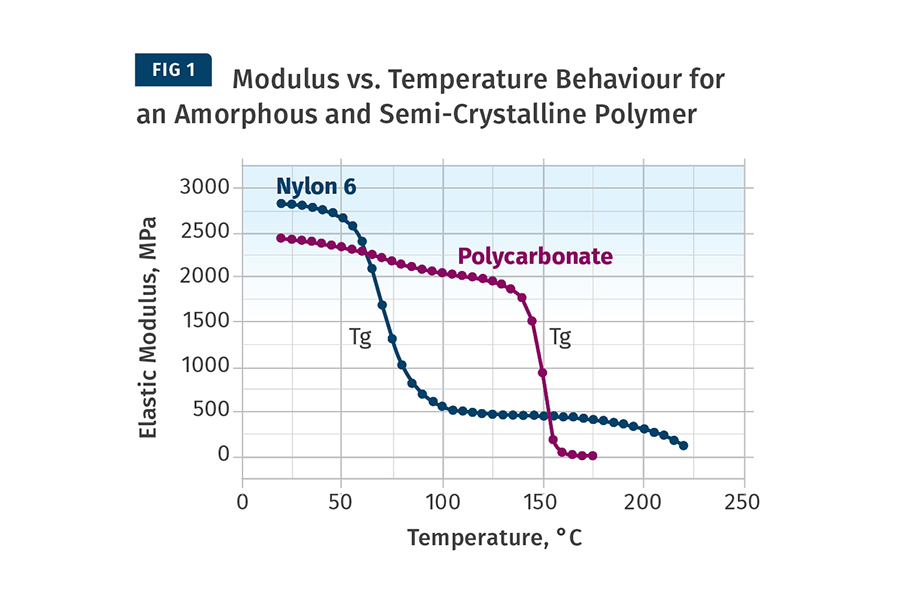
The difference between amorphous & semi-crystalline film. Semi-crystalline films have a highly ordered molecular structure with sharp melt points. While amorphous polymers soften gradually when the temperature rises, semi-crystalline plastics do not. Instead, they remain solid until a certain quantity of heat is absorbed.

Sólidos cristalinos y amorfos explicación, diferencias, ejemplos, etc HiTech
Unique among polymers PVC, PE, PP and PS are general purpose plastics. The features of a plastic are determined by its chemical composition and type of molecular structure (crystalline or amorphous). PVC has an amorphous structure that is directly related to the polar chlorine atoms in its molecular structure.
Basic Approach for the selection of Engineering Plastics
It should be noted however, that with both the semi crystalline and amorphous materials at sufficiently high temperature (this is when the material is in its melt state) the molecular structure is amorphous. Table 6.1 classifies some common materials into these two groups.
PPT Amorphous Metal Alloys PowerPoint Presentation, free download ID6510039
Describe at least two ways to determine experimentally whether a material is crystalline or amorphous. 6. Explain why each characteristic would or would not favor the formation of an amorphous solid. a. slow cooling of pure molten material. b. impurities in the liquid from which the solid is formed.

Discussion Which One Of The Following Is Non Crystalline Or Amorphous Updated Sharing Place
Amorphous plastics typically have a shiny and smooth surface finish due to their non-crystalline structure. Some amorphous resins can transmit light which makes them well suited to aesthetic & optical applications. Shrinkage Amorphous plastics generally exhibit low and uniform shrinkage upon cooling.

Difference between the crystalline and amorphous phases [17]. Download Scientific Diagram
The arrangement of molecular chains in amorphous and semicrystalline polymers. Solidification from the melt Polymers are composed of long molecular chains which form irregular, entangled coils in the melt. Some polymers retain such a disordered structure upon freezing and readily convert into amorphous solids.

Courses nanoHUBU Primer on Semiconductor Fundamentals Fall 2018 (SelfPaced)
As a result, many polymers are semi-crystalline, with regions called lamellae where portions of chains have aligned parallel to each other, but also with large amorphous areas that are much more randomly oriented. As a result, a polymer sample might be 80% amorphous with only 20% of its chain lengths aligned in crystalline lamellae.
X ray diffraction of semi crystalline and amorphous in Polymer Download Scientific Diagram
The observed heat of fusion of the mass polymer varied considerably with crystallization temperature, but was in the range 3-5% crystalline, i.e. 0.35-0.55 kJ monomer mol - 1 g: Onset Figure 3 lb Min Isothermal crystallization of PVC Isothcrmat 410 K 2b Metting ~E I I 380 480 K Figure 4 Melting endotherms of PVC 986 POLYMER, 1980, Vol 21.
click to know DISTINCTION BETWEEN CRYSTALLINE AND AMORPHOUS SOLIDS
A crystalline polymer is a type of long-chain organic material characterized by the presence of lamellae, which are ordered zones of aligned molecular chains. Crystalline polymers have highly structured regions and can in some cases be entirely composed of a single crystal aligned on one axis. The degree of crystallinity and mutual alignment is.

Resources ECE 695A Lecture 5 Amorphous Material/Interfaces Watch Presentation
Polymers with an amorphous morphology have their atoms held together in a loose structure, but this structure is never orderly or predictable, which is why chemists will say that amorphous solids have no long-range order. To understand this better, think of a polymer chain as a piece of spaghetti.